Soils: the science
When designing your vegetable or flower garden it is important to know your soil pH . Whether your soil is clay, sand or loam most plants, including grass, vegetables and flowers grow best at a specific soil pH. Therefore when planning or designing a garden, be it a small cottage garden, a herb garden or even a large public garden understanding soil pH can help define your planting plan and help make the most of your site.
. Whether your soil is clay, sand or loam most plants, including grass, vegetables and flowers grow best at a specific soil pH. Therefore when planning or designing a garden, be it a small cottage garden, a herb garden or even a large public garden understanding soil pH can help define your planting plan and help make the most of your site.
There are many sources of reference available such as websites and books to advise on the soil pH most appropriate for growing particular plants. We have produced a leaflet, available below, to help you get started. In it you will find examples of the optimum soil pH for a variety of plants and the relationship between soil pH and plant nutrient availability.
Soil pH
pH is a term used to describe whether your soil is acidic, neutral or alkaline. Strictly speaking it is a measure of the concentration of the hydrogen ion (H+) in the moisture associated with your soil. It is measured on a scale of 0-14, where 1 is very acid and 14 is very alkali; typically soils are between pH 4 and 8.
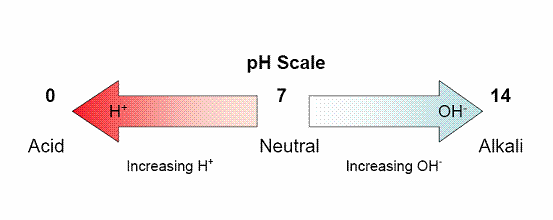
How is soil pH measured?
Soil test kits are available from garden centres. These commonly involve taking a soil sample and mixing it with a liquid which changes colour depending on the pH of the soil. They are generally not very accurate and can be fiddly to use. The biggest drawback is that the colour change can be masked by the colour of the soil, for example, a very peat soil can colour the solution a very dark brown. Typically only a very small soil sample is used so it is difficult to get a sample which is representative of your whole garden.
Needless to say there is a better way. In the laboratory we use a pH electrode. This approach uses a much bigger sample (mixed and sieved beforehand) so it is more representative and provides a definitive value using a carefully calibrated piece of analytical equipment.
Can I change the pH of my soil?
Often gardeners wish to alter the pH of their soil to improve the soil for planting different plants. Adding lime (ground limestone) to soil can increase the pH (reduce the number of H+ ions) and make your soil more suitable for growing certain plant species. This is often a good tactic if your soil has been repeatedly fertilised, which has a tendency to make your soil more acidic.
How much lime is required to raise my Soil pH?
The quantity of lime required to increase soil pH varies, typically clay soil requires more lime than a sandy soil. However, as a rough estimate you should apply 0.1 kg of ground limestone to a square metre of soil for an increase in soil pH of 0.1 pH units.
For example, to increase soil pH from 5.7 to 6 (a pH suitable for growing grass) would require 0.3 kg lime per square metre of soil. Note, it is important not to add too much lime at one time; generally the maximum is 0.4 kg per square metre. If you need to add more than 0.4 kg per square metre it’s best to do it over one or two years and test the soil between applications.
How do I spply lime to my soil?
Calculate how much lime is required for the size of area to be treated. Divide the pile into two halves. Walk across your garden from top to bottom (north to south) and using a scoop apply the lime in a semi circular casting action so that once at the bottom of the garden no lime is left and the area is evenly covered. Repeat the process using the other half of the lime; however walk from one side to the other (east to west), this will ensure an even coverage.
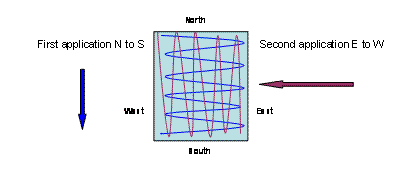
Typically lime is spread in the spring or autumn. It can be applied on top of grass or bare earth.
My soil is neutral or alkali can I make it acidic?
Soils derived from limestone or chalk are typically neutral or alkali. Although technically feasible to apply compounds, for example, aluminium sulphate, to decrease soil pH (make it more acidic), the quantities required are generally so high it is not cost effective. Most garden designers would recommend building raised gardens or growing in pots to counteract the natural alkalinity of the soil in those areas.
Available nutrients
Plants require certain elements for growth, for example, phosphorous (P) for root growth, potassium (K) for flowers and fruit, nitrogen (N) for foliage. The availability of these nutrients can effect plant growth. The concentrations of available nutrients describe whether additional fertilisers, containing these nutrients, are required. The nutrients can be applied from an inorganic, that is, manmade fertiliser or from additional organic, that is, natural, fertiliser, for example, manure, compost. Care must be taken not to increase the concentration of nutrients to the point where they become detrimental to plant growth.
Soil nitrate
Nitrogen is a major plant nutrient and can be found in soils in a variety of forms, some available to plants, for example, nitrate and some which are unavailable, for example, nitrogen associated with soil biomass or organic material. Soil nitrogen may change form, depending upon a variety of factors for example, soil water concentration, temperature, cropping. Generally a soil nitrate concentration of approximately 100 mg kg-1 maximises crop yields but due to the dynamic nature of soil nitrogen and complex interactions within the soil biota, this is only an estimate. Long term nitrogen fertility is best maintained by good soil structure and organic matter inputs, for example, manure.
Soil organic matter
The organic matter present within a soil has an influence on a variety of soil parameters; it helps bind soil particles together into water stable aggregates thus aiding texture and aeration, it acts as major source of plant nutrients, for example, phosphorous and in particular nitrogen, it effects the quantity of water a soil can store and acts as an energy source for the soil biota.



